- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

TMB Reference Standard
Background & Product Introduction
Tumor mutation burden (TMB) refers to the number of somatic mutations in the tumor genome after removing germline mutations. It is defined as the total number of somatic gene coding errors, base substitutions, gene insertions or deletions detected per million bases. The TMB value can reflect the potential for the production of new tumor antigens in the tumor and is closely related to DNA repair defects. In many tumors, dMMR and MSI-H patients have higher TMB.
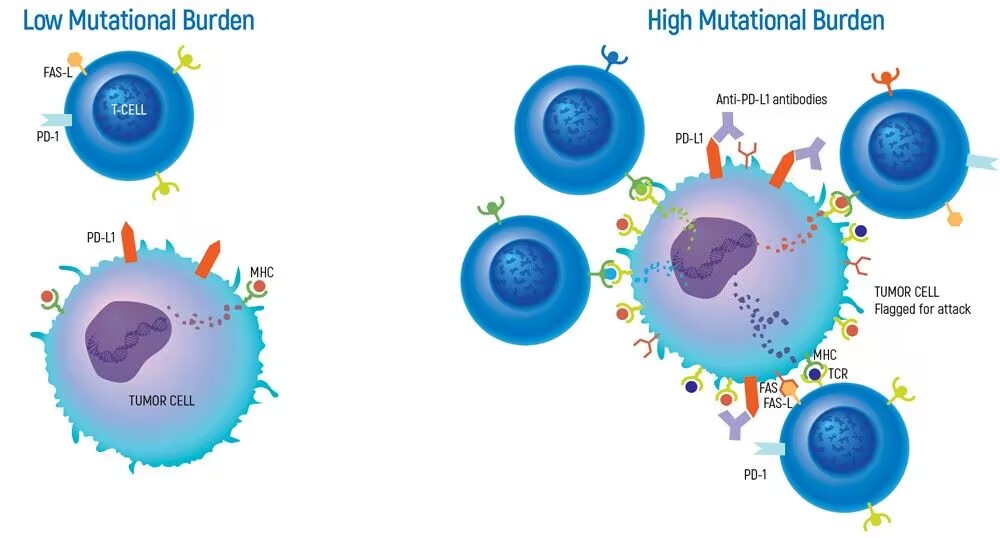
TMB measurement has become a critical biomarker in predicting immunotherapy response and guiding personalized cancer treatment. Our TMB Reference Standards address the urgent need for reliable quality control materials in next-generation sequencing (NGS) workflows. Developed using clinically validated mutation profiles, these standards precisely mimic real tumor genomic characteristics while maintaining batch-to-batch consistency across production lots.
CB-Gene has launched TMB standards to ensure the detection limit, sensitivity and stability of the diagnostic method.
Product Features
Calculate TMB score by WES sequencing compared with normal samples;
Evaluate detection limit by different tumor content percentages;
Evaluate detection limit by different AF cutoff values;
tTMB and bTMB samples;
Multiplex Compatibility: Validated for major NGS platforms (Illumina, Thermo Fisher, MGI Tech) and common panel sizes (500+ genes)
Applications
Testing the sensitivity, accuracy and specificity of experimental methods
Optimizing and validating new experimental procedures or kits
Daily QC monitoring of NGS library preparation and sequencing runs
| Product Name | Catalog No. | Details | Inquiry |
|---|---|---|---|
| Clinical-Grade tTMB Ref Std for Cancer I-O | CBP80001-1/2/3/4/5/6/7/8 | View detail » | Inquire |
| Genomic DNA tTMB-P9 Standard for NGS QC | CBP80001-9 | View detail » | Inquire |
| Dual ctDNA/gDNA TMB 22.91 Standard for I-O NGS | CBP80001-10 | View detail » | Inquire |