- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

HRD Reference Standard
Background
Homologous Recombination Repair (HRR) is one of the important ways to repair DNA double-strand damage, and the HRR pathway includes BRCA1/2 genes. Tumor cells with homologous recombination deficiency (HRD) are sensitive to platinum-containing chemotherapy and poly ADP-ribose polymerase inhibitor (PARPi).
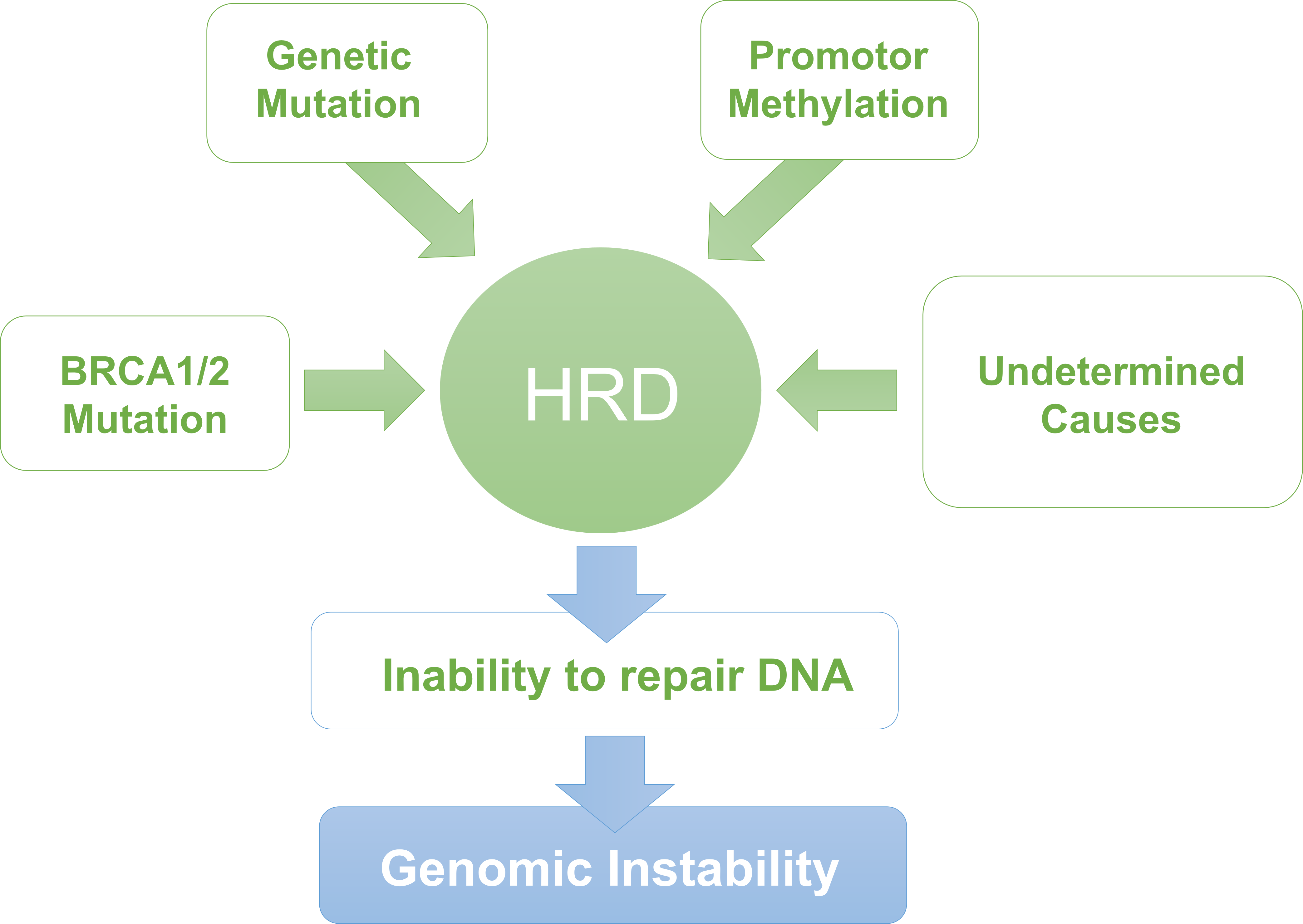
Homologous Recombination Deficiency (HRD) has emerged as a critical biomarker for predicting therapeutic response to PARP inhibitors in various cancers. To ensure the accuracy and reproducibility of HRD testing in clinical diagnostics and research, standardized reference materials are essential.
Key Features
Paired immortalized cell lines
Mimics authentic HRD-positive genomic characteristics
including: Pathogenic BRCA1/2 variants (germline/somatic) 、Genome-wide loss of heterozygosity (LOH) 、Telomeric allelic imbalance (TAI) 、Large-scale state transitions (LST)
Equipped with WGS data and analysis process, it can be used to verify the accuracy of the panel
Values are determined through WGS+ double sample analysis process
HRD score has a wide distribution range and can simulate real samples
Standard products in the form of gDNA/FFPE/ctDNA can be customized
Applications
Clinical assay validation for PARP inhibitor therapy selection
Laboratory quality control for HRD score calculation algorithms
Proficiency testing in molecular pathology workflows
Companion diagnostic development optimization
NGS platform performance benchmarking
| Product Name | Catalog No. | Details | Inquiry |
|---|---|---|---|
| Package-Ref™ HRD Cocktail Reference Standard | CBP90023 | View detail » | Inquire |