- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

CNV Reference Standard
Background
Copy number variation (CNV) refers to genomic structural alterations involving DNA segments ≥1 kb, playing a pivotal role in hereditary disease research, cancer biomarker discovery, and precision medicine. However, inconsistent CNV detection results across platforms and laboratories persist due to the lack of universally accepted reference materials. Our CNV reference standard effectively addresses this challenge by providing traceable, multi-platform validated genomic control samples.
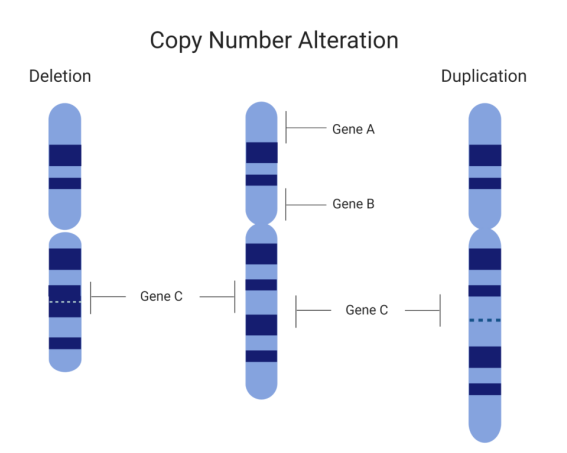
Detection of CNV copy number variation
Multiple technologies are employed for CNV detection, including fluorescence in situ hybridization (FISH), quantitative PCR (qPCR), multiplex ligation-dependent probe amplification (MLPA), single-nucleotide polymorphism arrays (SNP arrays), and next-generation sequencing (NGS).
Reliable reference materials are critical for calibrating these platforms and ensuring consistent, accurate results across laboratories. To meet this need, CB-Gene has developed a comprehensive portfolio of CNV reference standards for key tumor-associated genes, providing validated controls essential for assay development, validation, and routine quality control.
Key Features
Biologically Relevant Source: Derived from engineered tumor cell lines to authentically mimic the genomic profile of patient tumors.
Flexible Formats: Available as high-quality genomic DNA (gDNA), including formats compatible with FFPE-simulated workflows.
Digitally PCR-Quantified: Every target's copy number is precisely certified by ddPCR, guaranteeing accuracy and traceability.
Comprehensive Gene Coverage: Includes a wide array of critical targets, such as common proto-oncogenes and tumor suppressor genes.