- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBP90023
CBP90023
| Availability: | |
|---|---|
Homologous Recombination Deficiency (HRD) makes tumor cells sensitive to platinum drugs and PARP inhibitors—key therapies for ovarian, breast, and other cancers. The Package-Ref™ HRD Cocktail Reference Standard (CBP90023) is derived from paired tumor-normal cell lines, evaluated via Whole-Genome Sequencing (WGS) to assign precise HRD scores. It addresses unmet needs for IVD enterprises, labs, and quality assessment programs, serving as a reference for kit validation, positive/negative controls, and inter-lab comparisons.
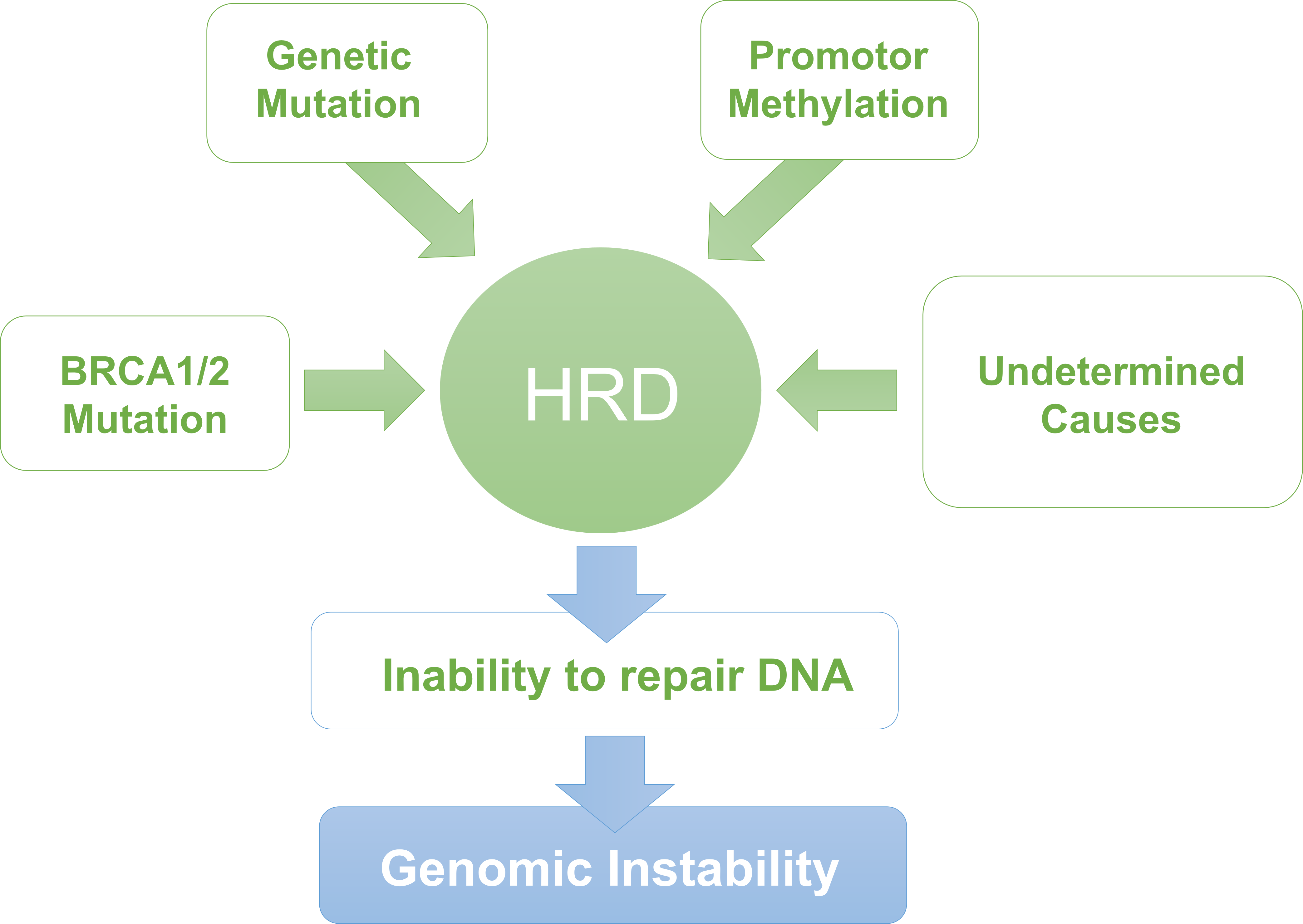
Unlike single-sample standards, its paired (tumor-normal) format enables accurate assessment of somatic HRD events, critical for distinguishing tumor-specific defects from germline variants.
Covering high, median, and low HRD scores, it validates an assay’s ability to correctly classify HRD status—essential for identifying patients who will benefit from PARP inhibitors.
With 3 years of stability at 2–8°C, it eliminates frequent reordering and ensures consistent QC results across months or years.
WGS evaluation guarantees accurate HRD score assignment, making it a trusted reference for comparing panel performance (e.g., HRD panel vs. WGS data).
We help you select the right HRD sample set (e.g., Positive-High for PARP inhibitor research, Negative-Low for specificity testing) based on your assay goals.
Upon delivery, you receive direct access to downloadable COAs for each paired sample (e.g., CBP90023-1N/CBP90023-1T) with detailed HRD scores, Concentration, and DNA electrophoresis.
Our team assists with designing validation experiments (e.g., testing LOH, LST, TAI—key HRD markers) and interpreting results to ensure your assay meets performance benchmarks.
For labs participating in external quality assessment (EQA), we provide data alignment support to ensure your results match industry standards.
HRD testing is increasingly central to precision oncology, but inconsistent standards lead to unreliable results. This product’s paired design, stratified scores, and WGS validation make it the ideal tool for validating HRD assays, monitoring daily QC, and complying with IVD development requirements. Request a sample today to advance your tumor therapy response testing.
Name | Package-Ref™ HRD Cocktail Reference Standard |
Cat. No. | CBP90023 |
Format | Genomic DNA |
Size | 1ug/vial * 2 vial |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | 2-8°C |
Expiry | 36 months from the date of manufacture |
Catalog ID | Sample | Match | HRD Score | Drug sensitivity | HRR | Remarks | COA |
CBP90023-1 | HRD-P1 | 0.86 | Positive -High | 78.4 | 500x WES | Paired samples | |
CBP90023-2 | HRD-P2 | 0.84 | Positive -High | 33.73 | 500x WES | Paired samples |
|
CBP90023-9 | HRD-P9 | 0.9 | Positive-High | N/A | 500x WES | Paired samples | |
CBP90023-3 | HRD-P3 | 0.8 | Positive-High | 144.54 | 500x WES | Paired samples |
|
CBP90023-4 | HRD-P4 | 0.83 | Critical Value-Median | 67.9 | 500x WES | Paired samples | |
CBP90023-15 | HRD-P15 | 0.88 | Critical Value-Median | N/A | 500x WES | Paired samples | |
CBP90023-10 | HRD-P10 | 0.92 | Negative-Low | 100 | 500x WES | Paired samples | |
CBP90023-13 | HRD-P13 | 0.95 | Negative-Low | 2.14 | 500x WES | Paired samples |
Homologous Recombination Deficiency (HRD) makes tumor cells sensitive to platinum drugs and PARP inhibitors—key therapies for ovarian, breast, and other cancers. The Package-Ref™ HRD Cocktail Reference Standard (CBP90023) is derived from paired tumor-normal cell lines, evaluated via Whole-Genome Sequencing (WGS) to assign precise HRD scores. It addresses unmet needs for IVD enterprises, labs, and quality assessment programs, serving as a reference for kit validation, positive/negative controls, and inter-lab comparisons.

Unlike single-sample standards, its paired (tumor-normal) format enables accurate assessment of somatic HRD events, critical for distinguishing tumor-specific defects from germline variants.
Covering high, median, and low HRD scores, it validates an assay’s ability to correctly classify HRD status—essential for identifying patients who will benefit from PARP inhibitors.
With 3 years of stability at 2–8°C, it eliminates frequent reordering and ensures consistent QC results across months or years.
WGS evaluation guarantees accurate HRD score assignment, making it a trusted reference for comparing panel performance (e.g., HRD panel vs. WGS data).
We help you select the right HRD sample set (e.g., Positive-High for PARP inhibitor research, Negative-Low for specificity testing) based on your assay goals.
Upon delivery, you receive direct access to downloadable COAs for each paired sample (e.g., CBP90023-1N/CBP90023-1T) with detailed HRD scores, Concentration, and DNA electrophoresis.
Our team assists with designing validation experiments (e.g., testing LOH, LST, TAI—key HRD markers) and interpreting results to ensure your assay meets performance benchmarks.
For labs participating in external quality assessment (EQA), we provide data alignment support to ensure your results match industry standards.
HRD testing is increasingly central to precision oncology, but inconsistent standards lead to unreliable results. This product’s paired design, stratified scores, and WGS validation make it the ideal tool for validating HRD assays, monitoring daily QC, and complying with IVD development requirements. Request a sample today to advance your tumor therapy response testing.
Name | Package-Ref™ HRD Cocktail Reference Standard |
Cat. No. | CBP90023 |
Format | Genomic DNA |
Size | 1ug/vial * 2 vial |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | 2-8°C |
Expiry | 36 months from the date of manufacture |
Catalog ID | Sample | Match | HRD Score | Drug sensitivity | HRR | Remarks | COA |
CBP90023-1 | HRD-P1 | 0.86 | Positive -High | 78.4 | 500x WES | Paired samples | |
CBP90023-2 | HRD-P2 | 0.84 | Positive -High | 33.73 | 500x WES | Paired samples |
|
CBP90023-9 | HRD-P9 | 0.9 | Positive-High | N/A | 500x WES | Paired samples | |
CBP90023-3 | HRD-P3 | 0.8 | Positive-High | 144.54 | 500x WES | Paired samples |
|
CBP90023-4 | HRD-P4 | 0.83 | Critical Value-Median | 67.9 | 500x WES | Paired samples | |
CBP90023-15 | HRD-P15 | 0.88 | Critical Value-Median | N/A | 500x WES | Paired samples | |
CBP90023-10 | HRD-P10 | 0.92 | Negative-Low | 100 | 500x WES | Paired samples | |
CBP90023-13 | HRD-P13 | 0.95 | Negative-Low | 2.14 | 500x WES | Paired samples |
General Information
Name | Package-Ref™ HRD Cocktail Reference Standard |
Cat. No. | CBP90023 |
Format | Genomic DNA |
Size | 1ug/vial * 2 vial |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | 2-8°C |
Expiry | 36 months from the date of manufacture |
General Information
Name | Package-Ref™ HRD Cocktail Reference Standard |
Cat. No. | CBP90023 |
Format | Genomic DNA |
Size | 1ug/vial * 2 vial |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | 2-8°C |
Expiry | 36 months from the date of manufacture |
Detailed Data
Catalog ID | Sample | Match | HRD Score | Drug sensitivity | HRR | Remarks | COA |
CBP90023-1 | HRD-P1 | 0.86 | Positive -High | 78.4 | 500x WES | Paired samples | |
CBP90023-2 | HRD-P2 | 0.84 | Positive -High | 33.73 | 500x WES | Paired samples |
|
CBP90023-9 | HRD-P9 | 0.9 | Positive-High | N/A | 500x WES | Paired samples | |
CBP90023-3 | HRD-P3 | 0.8 | Positive-High | 144.54 | 500x WES | Paired samples |
|
CBP90023-4 | HRD-P4 | 0.83 | Critical Value-Median | 67.9 | 500x WES | Paired samples | |
CBP90023-15 | HRD-P15 | 0.88 | Critical Value-Median | N/A | 500x WES | Paired samples | |
CBP90023-10 | HRD-P10 | 0.92 | Negative-Low | 100 | 500x WES | Paired samples | |
CBP90023-13 | HRD-P13 | 0.95 | Negative-Low | 2.14 | 500x WES | Paired samples |
Detailed Data
Catalog ID | Sample | Match | HRD Score | Drug sensitivity | HRR | Remarks | COA |
CBP90023-1 | HRD-P1 | 0.86 | Positive -High | 78.4 | 500x WES | Paired samples | |
CBP90023-2 | HRD-P2 | 0.84 | Positive -High | 33.73 | 500x WES | Paired samples |
|
CBP90023-9 | HRD-P9 | 0.9 | Positive-High | N/A | 500x WES | Paired samples | |
CBP90023-3 | HRD-P3 | 0.8 | Positive-High | 144.54 | 500x WES | Paired samples |
|
CBP90023-4 | HRD-P4 | 0.83 | Critical Value-Median | 67.9 | 500x WES | Paired samples | |
CBP90023-15 | HRD-P15 | 0.88 | Critical Value-Median | N/A | 500x WES | Paired samples | |
CBP90023-10 | HRD-P10 | 0.92 | Negative-Low | 100 | 500x WES | Paired samples | |
CBP90023-13 | HRD-P13 | 0.95 | Negative-Low | 2.14 | 500x WES | Paired samples |
Product Application
1.Evaluate the stability of the experiment and analysis process
2.Test whether each independent mutation of HRD-related LOH, LST, and TAI can be accurately judged
3.Compare WGS data with HRD panel to test panel design
4.Different mixing ratios of tumor and wild type can be used to determine the detection limit of each mutation and determine the minimum tumor ratio
5.Negative and negative reference/quality control products
Product Application
1.Evaluate the stability of the experiment and analysis process
2.Test whether each independent mutation of HRD-related LOH, LST, and TAI can be accurately judged
3.Compare WGS data with HRD panel to test panel design
4.Different mixing ratios of tumor and wild type can be used to determine the detection limit of each mutation and determine the minimum tumor ratio
5.Negative and negative reference/quality control products