- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

Views: 0 Author: Site Editor Publish Time: 2025-09-23 Origin: Site
Background In the field of eugenics and good parenting, advances in prenatal testing technology have brought hope and peace of mind to countless families. Non-invasive prenatal testing (NIPT), a revolutionary technology, has rapidly become the preferred method for prenatal screening due to its high accuracy, safety, and convenience. Before the advent of NIPT, prenatal testing primarily relied on invasive methods such as amniocentesis and chorionic villus sampling (CVS). While these methods offer high accuracy, they carry certain risks. To overcome these limitations, scientists began exploring safer and more convenient testing methods. In 1997, scientists discovered the presence of cell-free fetal DNA (cfDNA) in maternal peripheral blood, laying the foundation for non-invasive prenatal testing. In 2011, NIPT was first used clinically, ushering in a new era in prenatal testing. In the early stage, CB-Gene Bio has launched NIPT standard products to assist non-invasive prenatal testing. Genetic testing is a crucial piece in the complex puzzle of fertility and childbearing, and paired samples with varying fetal fractions play a crucial role, particularly in the field of non-invasive prenatal testing (NIPT). Cell-free DNA (cfDNA) in maternal plasma is a mixture of maternal and fetal DNA. The proportion of fetal DNA in cfDNA varies, typically ranging from 5% to 40%, influenced by numerous factors, such as gestational age and placental condition. Paired samples with varying fetal fractions can accurately simulate this complex situation in real-life pregnancies. For example, paired samples with a low fetal fraction (e.g., 5%-10%) can simulate early pregnancy or certain special circumstances, when fetal DNA content is low; whereas paired samples with a high fetal fraction (e.g., 30%-40%) correspond to late pregnancy or well-developed placentation, when fetal DNA is relatively abundant. By using these samples, testing laboratories can more realistically evaluate the performance of testing technologies in various clinical scenarios. New Product Highlights When developing new NIPT testing technologies or upgrading existing ones, paired samples with varying fetal DNA ratios are essential evaluation tools. By analyzing the degree of match between test results and known sample genetic information, key metrics such as accuracy, false positive rate, and false negative rate can be assessed for varying fetal DNA content, thereby determining the technology's maturity and potential for further optimization. Paired samples with varying fetal DNA ratios for eugenics and prenatal care include paired positive references, paired negative references, and paired detection limit references at varying ratios. This comprehensive system acts as a precise calibrator tailored to the testing process. Quantitative analysis is crucial in genetic testing, especially for accurately determining key metrics such as fetal concentration and the number of chromosomal abnormality copies. Paired samples enable precise control of the incorporation ratio of fragmented DNA through ddPCR, allowing for the establishment of varying fetal DNA ratios. In contrast, unpaired samples are difficult to achieve such precise quantification. In the field of eugenics and prenatal care genetic testing, paired samples with varying fetal DNA ratios, with their unique design and sophisticated preparation process, have become key elements in improving test accuracy, optimizing testing processes, and assisting clinical diagnosis, contributing significantly to safeguarding the genetic health of newborns. Product Good fertility and good parenting are the aspirations of every family and a shared responsibility of society. The eugenics and good parenting paired standard products launched by CoBio provide strong support for precise testing and scientific intervention. Let us work together to safeguard the health and future of every new life with the power of science! 产品清单 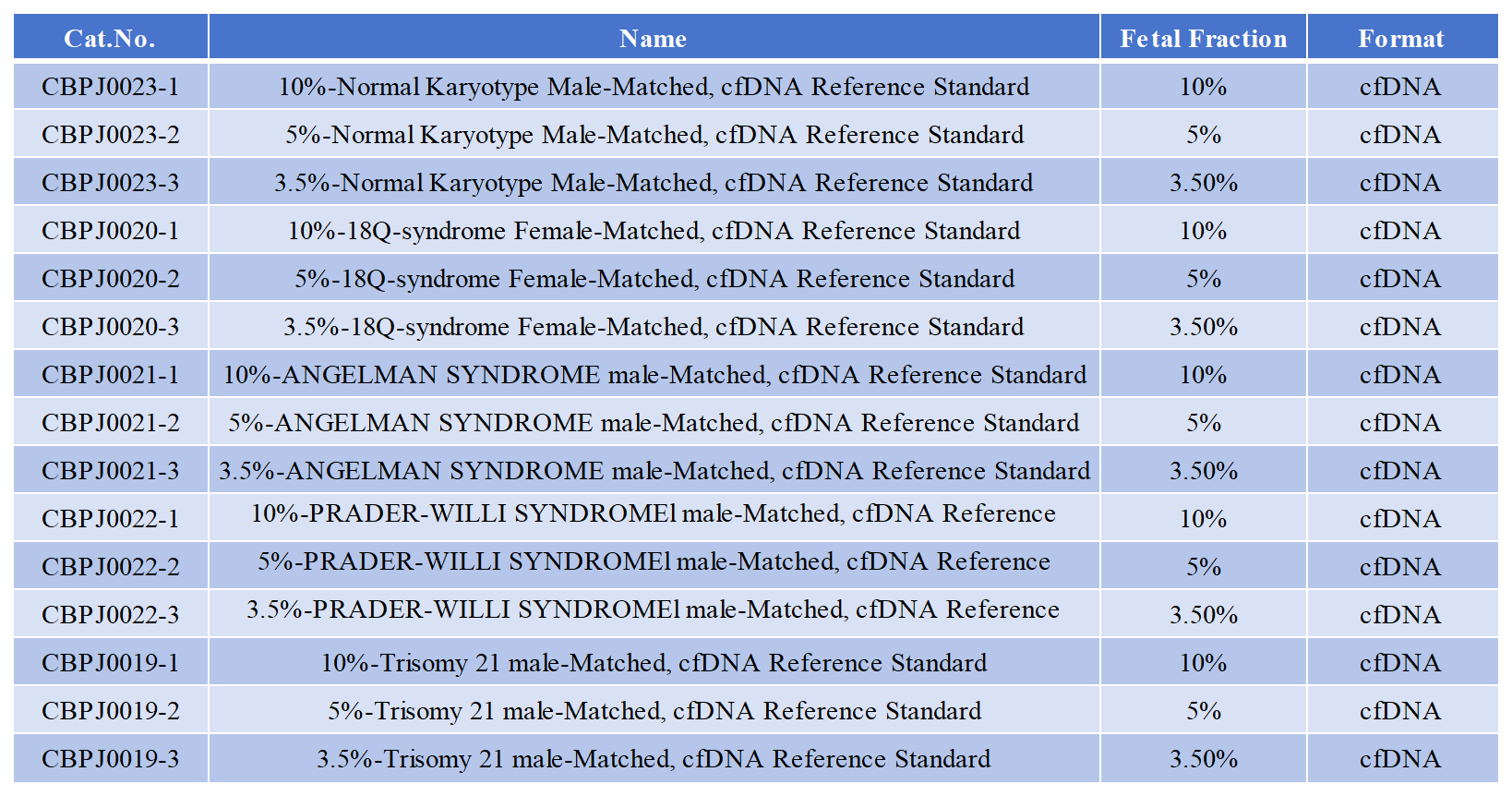

This category is empty.