- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPN0001
CBPN0001
| Availability: | |
|---|---|
Introduction
CLDN18.2 (Claudin 18.2) is a key member of the tight junction protein family, specifically expressed in differentiated gastric mucosal epithelial cells.It demonstrates consistent and prevalent overexpression across multiple solid tumors—including gastric, esophageal, and pancreatic cancers. This expression persists not only in primary lesions but also in metastatic sites, making CLDN18.2 as a promising novel biomarker in targeted cancer therapy and diagnostics. To ensure accurate and reproducible immunohistochemical (IHC) detection results for CLDN18.2, highly standardized quality control materials are essential throughout the experimental process. To meet this need, we introduce the Positive(+++) FFPE Slide Reference Standard. This standard is engineered for IHC assay validation, protocol optimization, and routine laboratory quality control. It is suitable for antibody performance validation, experimental workflow standardization, and internal laboratory quality control, ultimately aiding in reliable tumor pathology diagnosis and targeted drug development.
Product Features
1.Defined Strong Positive (+++) Expression: Provides a stable, homogeneous high-level antigen expression for CLDN18.2 IHC. This facilitates clear microscopic interpretation by users and establishes a positive control standard.
2.High-quality Tissue Sections: Using standardized paraffin embedding, our tissue sections are precision-cut to a consistent 4μm thickness. This process ensures complete tissue morphology and clear cellular structures, which meets and exceeds the section specifications for international IHC testing.
3.Rigorous Quality Control Process: Each batch of reference standards undergoes strict IHC staining detection to ensure consistent CLDN18.2 expression intensity, minimal batch-to-batch variation, and enhanced result reliability.
4.Guaranteed Stability & Shelf Life: With a 36-month shelf life under recommended storage conditions (-25°C to -15°C). This ensures no performance degradation occurs within the validity period, facilitating long-term laboratory use and comparative analysis.
5.Ready-to-Use Design:Provided as pre-cut paraffin sections, these standards require no preprocessing. They can be stained directly following standard IHC protocols upon opening, saving time and reducing operational errors.
Product Advantages
1.Enhancing Experimental Reliability: Serves as an internal QC reference to monitor all critical IHC steps—including antigen retrieval, antibody incubation, and development. This allows for timely identification of issues and improves testing accuracy.
2.Standardized Support Method: Enables process standardization and result comparison across different laboratories, facilitating data consistency in multicenter studies or clinical trials.
3.Optimized Antibody Evaluation: Provides an clear positive benchmark during antibody screening, titration, and validation, assist users in assessing antibody sensitivity and specificity.
4.Broad IHC Platform Compatibility: Suitable for automated or manual IHC staining systems, compatible with a wide range of commercial detection kits, offering flexible application.
5.Professional Technical Support: Provide detailed product technical documentation and storage usage guidelines, and offer relevant application recommendations tailored to your specific experimental requirements.
Primary Applications
This product is primarily intended as an IHC QC reference standard for research and is suitable for the following scenarios:
*Tumor Research Field: A positive control reference standard for IHC detection in pathological studies of CLDN18.2-related tumors such as gastric cancer, esophageal cancer, and pancreatic cancer.
*Support Drug Development: Serves as a standardized tool for tissue testing in preclinical and clinical trials of CLDN18.2-targeted therapeutics (such as monoclonal antibodies, bispecific antibodies, ADCs, etc.).
*Establish Laboratory QC System: Assist in establishing and maintaining a QC system for pathology departments and R&D laboratories, ensuring consistent daily test results.
*Antibody and Reagent Performance Validation: Evaluating the performance of CLDN18.2 antibodies or IHC detection reagents from different manufacturers, assisting laboratories in selecting the optimal detection protocol.
*Teaching and Training: Educational specimens for IHC interpretation in pathology technician training, assisting trainees in mastering criteria for assessing positive staining intensity.
Why Choose Us
1.Target-Specific Focus: The product is tailored to the expression characteristics of CLDN18.2, utilizing tissues with high expression levels that have undergone rigorous validation to ensure its representativeness and authority as a reference standard.
2.Consistent and Reliable Quality: We implement standardized procedures across every step—from tissue sourcing and processing to sectioning and finial QC —minimizing batch-to-batch variation and ensuring reproducibility in every experiment.
3.User-Friendly Design: Ready-to-use slice design combined with clear storage conditions and extended shelf life facilitates laboratory planning, usage, and management while minimizing waste.
4.Advancing Precision Medicine: As CLDN18.2 emerges as a key therapeutic target, this reference standard is critical for standardizing detection assays. It provides robust support for precise diagnosis and treatment evaluation.
5.Enabling Precision Medicine: It provides the reliability necessary for accurate patient stratification and treatment response evaluation.
6.Professional Service : We are committed to providing high-quality tools and support for scientific research and preclinical studies, and offer specialized technical consultation to meet user’s requirements.
Frequently Asked Questions (FAQ)
What is the correct step of using this reference standard?
It is recommended that this product be stained simultaneously with the tissue sections to be tested in each batch of IHC experiments. Following the laboratory's standard IHC protocol(dewaxing, antigen retrieval, antibody incubation, and detection and counterstaining). The validity of the experimental process can be determined by observing whether this product exhibits the expected strong positive staining.
How should the product be stored? Can it be reused after opening?
The product should be sealed at -25°C to -15°C, avoiding repeated freeze-thaw cycles. Each slide is typically recommended for single-use to ensure uniform staining conditions. If multiple uses are required, follow the cutting and storage conditions specified in the manual; however, this may affect staining results in subsequent applications.
What detection systems is this product compatible with?
This product is compatible with mainstream IHC detection systems (such as the HRP/DAB system). Users may use it as a positive control when using primary antibodies of different clonal numbers or detection kits from different manufacturers. However, optimal staining conditions may require minor adjustments based on the specific reagent.
Can this standard be used for fluorescence Immunofluorescence (IF) or other detection?
This product is primarily optimized and validated for conventional IHC (DAB staining). If used for IF, users are advised to first validate its fluorescence signal intensity and background characteristics within that detection system.
What is the purpose of the “sample” images provided with the product?
The sample image illustrates the typical morphology and staining intensity (+++) of this product, as observed under standard IHC staining. It serves as a reference standard for users to assess whether their experiments meet the expected quality control outcomes.
Introduction
CLDN18.2 (Claudin 18.2) is a key member of the tight junction protein family, specifically expressed in differentiated gastric mucosal epithelial cells.It demonstrates consistent and prevalent overexpression across multiple solid tumors—including gastric, esophageal, and pancreatic cancers. This expression persists not only in primary lesions but also in metastatic sites, making CLDN18.2 as a promising novel biomarker in targeted cancer therapy and diagnostics. To ensure accurate and reproducible immunohistochemical (IHC) detection results for CLDN18.2, highly standardized quality control materials are essential throughout the experimental process. To meet this need, we introduce the Positive(+++) FFPE Slide Reference Standard. This standard is engineered for IHC assay validation, protocol optimization, and routine laboratory quality control. It is suitable for antibody performance validation, experimental workflow standardization, and internal laboratory quality control, ultimately aiding in reliable tumor pathology diagnosis and targeted drug development.
Product Features
1.Defined Strong Positive (+++) Expression: Provides a stable, homogeneous high-level antigen expression for CLDN18.2 IHC. This facilitates clear microscopic interpretation by users and establishes a positive control standard.
2.High-quality Tissue Sections: Using standardized paraffin embedding, our tissue sections are precision-cut to a consistent 4μm thickness. This process ensures complete tissue morphology and clear cellular structures, which meets and exceeds the section specifications for international IHC testing.
3.Rigorous Quality Control Process: Each batch of reference standards undergoes strict IHC staining detection to ensure consistent CLDN18.2 expression intensity, minimal batch-to-batch variation, and enhanced result reliability.
4.Guaranteed Stability & Shelf Life: With a 36-month shelf life under recommended storage conditions (-25°C to -15°C). This ensures no performance degradation occurs within the validity period, facilitating long-term laboratory use and comparative analysis.
5.Ready-to-Use Design:Provided as pre-cut paraffin sections, these standards require no preprocessing. They can be stained directly following standard IHC protocols upon opening, saving time and reducing operational errors.
Product Advantages
1.Enhancing Experimental Reliability: Serves as an internal QC reference to monitor all critical IHC steps—including antigen retrieval, antibody incubation, and development. This allows for timely identification of issues and improves testing accuracy.
2.Standardized Support Method: Enables process standardization and result comparison across different laboratories, facilitating data consistency in multicenter studies or clinical trials.
3.Optimized Antibody Evaluation: Provides an clear positive benchmark during antibody screening, titration, and validation, assist users in assessing antibody sensitivity and specificity.
4.Broad IHC Platform Compatibility: Suitable for automated or manual IHC staining systems, compatible with a wide range of commercial detection kits, offering flexible application.
5.Professional Technical Support: Provide detailed product technical documentation and storage usage guidelines, and offer relevant application recommendations tailored to your specific experimental requirements.
Primary Applications
This product is primarily intended as an IHC QC reference standard for research and is suitable for the following scenarios:
*Tumor Research Field: A positive control reference standard for IHC detection in pathological studies of CLDN18.2-related tumors such as gastric cancer, esophageal cancer, and pancreatic cancer.
*Support Drug Development: Serves as a standardized tool for tissue testing in preclinical and clinical trials of CLDN18.2-targeted therapeutics (such as monoclonal antibodies, bispecific antibodies, ADCs, etc.).
*Establish Laboratory QC System: Assist in establishing and maintaining a QC system for pathology departments and R&D laboratories, ensuring consistent daily test results.
*Antibody and Reagent Performance Validation: Evaluating the performance of CLDN18.2 antibodies or IHC detection reagents from different manufacturers, assisting laboratories in selecting the optimal detection protocol.
*Teaching and Training: Educational specimens for IHC interpretation in pathology technician training, assisting trainees in mastering criteria for assessing positive staining intensity.
Why Choose Us
1.Target-Specific Focus: The product is tailored to the expression characteristics of CLDN18.2, utilizing tissues with high expression levels that have undergone rigorous validation to ensure its representativeness and authority as a reference standard.
2.Consistent and Reliable Quality: We implement standardized procedures across every step—from tissue sourcing and processing to sectioning and finial QC —minimizing batch-to-batch variation and ensuring reproducibility in every experiment.
3.User-Friendly Design: Ready-to-use slice design combined with clear storage conditions and extended shelf life facilitates laboratory planning, usage, and management while minimizing waste.
4.Advancing Precision Medicine: As CLDN18.2 emerges as a key therapeutic target, this reference standard is critical for standardizing detection assays. It provides robust support for precise diagnosis and treatment evaluation.
5.Enabling Precision Medicine: It provides the reliability necessary for accurate patient stratification and treatment response evaluation.
6.Professional Service : We are committed to providing high-quality tools and support for scientific research and preclinical studies, and offer specialized technical consultation to meet user’s requirements.
Frequently Asked Questions (FAQ)
What is the correct step of using this reference standard?
It is recommended that this product be stained simultaneously with the tissue sections to be tested in each batch of IHC experiments. Following the laboratory's standard IHC protocol(dewaxing, antigen retrieval, antibody incubation, and detection and counterstaining). The validity of the experimental process can be determined by observing whether this product exhibits the expected strong positive staining.
How should the product be stored? Can it be reused after opening?
The product should be sealed at -25°C to -15°C, avoiding repeated freeze-thaw cycles. Each slide is typically recommended for single-use to ensure uniform staining conditions. If multiple uses are required, follow the cutting and storage conditions specified in the manual; however, this may affect staining results in subsequent applications.
What detection systems is this product compatible with?
This product is compatible with mainstream IHC detection systems (such as the HRP/DAB system). Users may use it as a positive control when using primary antibodies of different clonal numbers or detection kits from different manufacturers. However, optimal staining conditions may require minor adjustments based on the specific reagent.
Can this standard be used for fluorescence Immunofluorescence (IF) or other detection?
This product is primarily optimized and validated for conventional IHC (DAB staining). If used for IF, users are advised to first validate its fluorescence signal intensity and background characteristics within that detection system.
What is the purpose of the “sample” images provided with the product?
The sample image illustrates the typical morphology and staining intensity (+++) of this product, as observed under standard IHC staining. It serves as a reference standard for users to assess whether their experiments meet the expected quality control outcomes.
General Information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
General Information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 4μm |
Example |
|
Technical Data
Slice thickness | 4μm |
Example |
|
IHC Staining Results
 | 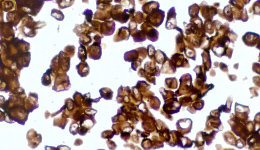 | 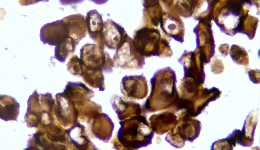 |
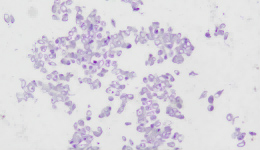 | 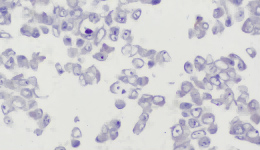 | 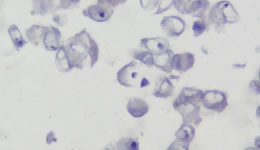 |
IHC Staining Results
 |  |  |
 |  |  |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |