- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPN0021
CBPN0021
| Availability: | |
|---|---|
The 2-in-1 ALK FFPE Slide IHC Reference Standard (hereafter referred to as "the Product") is a specialized quality control tool designed for immunohistochemistry (IHC) detection of ALK (Anaplastic Lymphoma Kinase) protein. It integrates two functional components—ALK-positive and ALK-negative cell fractions—into a single formalin-fixed, paraffin-embedded (FFPE) slide. This all-in-one design eliminates the need for separate positive and negative control slides, providing a streamlined solution for verifying the accuracy and reliability of ALK IHC assays in pathological laboratories.
Storage Condition: Maintains stable performance for up to 3 years when stored at -20°C (avoid repeated freeze-thaw cycles to prevent sample degradation).
Compatibility Scope: Fully adapts to mainstream automated IHC platforms, including but not limited to Leica Bond, Roche Ventana, and Dako Omnis systems.
Detection Adaptability: Supports multiple chromogenic systems, such as 3,3'-Diaminobenzidine (DAB) for visible brown staining and fluorescence-based detection methods for fluorescent signal readout.
Sample Uniformity: Each slide undergoes strict quality control to ensure consistent distribution of ALK-positive and negative cells, minimizing batch-to-batch variations.
Enables "ready-to-use" functionality: No additional steps like manual sectioning or pre-labeling are required. Laboratories can directly load the slide into IHC instruments, reducing quality control preparation time by 50% compared to self-made in-house controls.
Simplifies workflow management: Combining two control components in one slide reduces the number of slides to handle, label, and store, lowering the risk of sample mix-ups.
Features long-term stability: Unlike self-made controls that are prone to degradation within weeks, the Product retains its immunoreactivity for 3 years under proper storage, ensuring reliable results across multiple test batches.
Strict batch-to-batch quality control: Each production batch is tested against standardized ALK IHC protocols to confirm consistent signal intensity of positive cells and absence of non-specific staining in negative cells, avoiding false-positive or false-negative errors caused by control instability.
Works seamlessly with most commercial ALK primary antibodies: No need for protocol adjustments when switching between antibody brands, reducing the time spent on method optimization.
Adapts to both routine and advanced IHC workflows: Compatible with standard DAB staining for clinical diagnosis and fluorescence-based detection for high-sensitivity research applications, meeting diverse laboratory needs.
Aids in meeting laboratory quality standards: Provides a standardized reference for internal quality control (IQC), helping laboratories comply with medical laboratory management regulations (e.g., ISO 15189) and pass CNAS (China National Accreditation Service for Conformity Assessment) certification.
Facilitates inter-laboratory comparison (ILC): Uniform sample composition ensures consistent control performance across different laboratories, making it easier to identify deviations caused by instrument errors or operational inconsistencies.
Application scenario: Mandatory verification before the first use of a new ALK primary antibody or reagent batch.
Operation steps:
Run the Product in parallel with the new antibody/reagent following the standard ALK IHC protocol.
Evaluate the signal intensity of ALK-positive cells (should meet the antibody’s specified sensitivity) and check for cross-reactivity in ALK-negative cells (no non-specific staining allowed).
Only proceed with clinical sample testing if the validation results meet the quality criteria.
Application scenario: Synchronous quality control for every batch of ALK IHC tests (e.g., daily or per-sample-batch testing).
Operation steps:
Include the Product as a control slide in each IHC run (alongside clinical samples).
After staining, confirm that ALK-positive cells show clear, consistent staining (no signal loss) and ALK-negative cells remain unstained.
If abnormal results occur (e.g., weak positive signal or false negative in controls), stop sample testing immediately and troubleshoot (e.g., check instrument temperature, antibody expiration).
Application scenario: Collaborative quality assessment among multiple laboratories (e.g., hospital laboratory networks or EQA [External Quality Assessment] programs).
Operation steps:
Distribute the same batch of the Product to participating laboratories.
All laboratories test the slide using their in-house ALK IHC protocols.
Compare staining results across laboratories to identify problematic links (e.g., improper antigen retrieval conditions, expired reagents) and standardize operational procedures.
Application scenario: Training for new laboratory technicians on ALK IHC result interpretation.
Operation steps:
Use the Product as a "standard reference slide" to demonstrate typical ALK-positive (e.g., membrane/cytoplasmic staining) and negative (no staining) patterns.
Guide trainees to practice distinguishing true positive signals from non-specific background, helping them master judgment criteria (e.g., staining intensity, localization) quickly.
The Product must be stored at -20°C in a sealed container to prevent moisture absorption. Avoid repeated freeze-thaw cycles—once thawed, use it within 24 hours (if temporarily stored at 2-8°C) to avoid sample degradation. Do not store at room temperature for more than 4 hours.
Yes, the Product requires the same antigen retrieval step as clinical FFPE samples (e.g., heat-induced epitope retrieval with citrate buffer or EDTA buffer). Follow your laboratory’s standard ALK IHC protocol for antigen retrieval—no special adjustments are needed.
It is compatible with both manual and automated IHC staining. For manual staining, ensure consistent timing for antibody incubation and washing (as with clinical samples) to maintain control accuracy. For automated platforms, select the protocol matching your instrument model (e.g., Leica Bond’s ALK-specific program).
First, check if the storage conditions are correct (e.g., whether the slide was exposed to room temperature for too long). If storage is normal, verify the antibody expiration date and antigen retrieval parameters (e.g., temperature, time). If the issue persists, contact technical support for batch-specific quality verification.
No, reuse is not recommended. After one IHC staining cycle, the slide’s cell structure and antigenicity may be damaged, and residual antibodies or chromogens can cause cross-contamination in subsequent tests, leading to inaccurate results.
We possess a large-scale cell resource library, enabling precise selection of ALK-positive cell lines with stable expression and ALK-negative cell lines with no background expression—laying a solid foundation for consistent control performance.
Our mature FFPE slide preparation technology ensures intact cell morphology and preserved antigenicity. Each slide undergoes strict quality checks (e.g., hematoxylin-eosin staining for morphology, IHC validation for signal specificity) before leaving the factory.
Beyond the 2-in-1 ALK FFPE Slide, we offer a full range of IHC reference standards covering CLDN18.2, EGFR, HER2, NTRK, and other key targets. This allows laboratories to source all necessary controls from a single supplier, simplifying procurement and quality management.
We continuously develop reference standards for cutting-edge biomarkers (e.g., emerging immunotherapy targets), helping laboratories keep pace with advances in precision medicine.
Our technical team consists of experienced IHC specialists who provide one-on-one support, including protocol optimization, troubleshooting for abnormal control results, and customized training for laboratory staff.
We offer a product quality guarantee: If the slide fails to meet the specified performance standards under correct storage and use, we provide free replacement or full refund—reducing your risk.
Our IHC reference standards have been adopted by hundreds of pathological laboratories and participated in multiple national EQA programs, demonstrating reliable performance and compliance with clinical diagnostic requirements.
We adhere to strict manufacturing standards, with each product batch accompanied by a detailed quality inspection report—ensuring traceability and transparency for your quality control records.
General information
Name | ALK Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0021 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
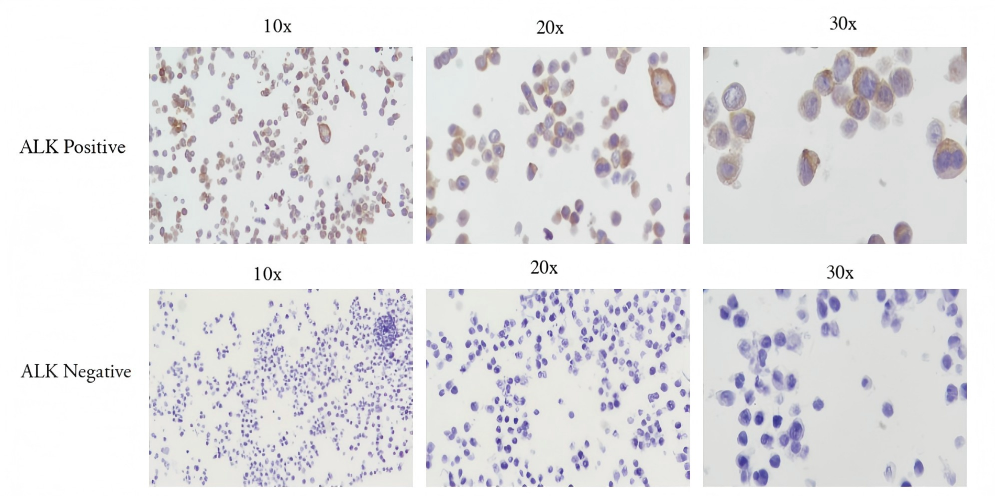
IHC staining results
Related Products List
Name | Catalog No. | Details |
ALK Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0022 |
The 2-in-1 ALK FFPE Slide IHC Reference Standard (hereafter referred to as "the Product") is a specialized quality control tool designed for immunohistochemistry (IHC) detection of ALK (Anaplastic Lymphoma Kinase) protein. It integrates two functional components—ALK-positive and ALK-negative cell fractions—into a single formalin-fixed, paraffin-embedded (FFPE) slide. This all-in-one design eliminates the need for separate positive and negative control slides, providing a streamlined solution for verifying the accuracy and reliability of ALK IHC assays in pathological laboratories.
Storage Condition: Maintains stable performance for up to 3 years when stored at -20°C (avoid repeated freeze-thaw cycles to prevent sample degradation).
Compatibility Scope: Fully adapts to mainstream automated IHC platforms, including but not limited to Leica Bond, Roche Ventana, and Dako Omnis systems.
Detection Adaptability: Supports multiple chromogenic systems, such as 3,3'-Diaminobenzidine (DAB) for visible brown staining and fluorescence-based detection methods for fluorescent signal readout.
Sample Uniformity: Each slide undergoes strict quality control to ensure consistent distribution of ALK-positive and negative cells, minimizing batch-to-batch variations.
Enables "ready-to-use" functionality: No additional steps like manual sectioning or pre-labeling are required. Laboratories can directly load the slide into IHC instruments, reducing quality control preparation time by 50% compared to self-made in-house controls.
Simplifies workflow management: Combining two control components in one slide reduces the number of slides to handle, label, and store, lowering the risk of sample mix-ups.
Features long-term stability: Unlike self-made controls that are prone to degradation within weeks, the Product retains its immunoreactivity for 3 years under proper storage, ensuring reliable results across multiple test batches.
Strict batch-to-batch quality control: Each production batch is tested against standardized ALK IHC protocols to confirm consistent signal intensity of positive cells and absence of non-specific staining in negative cells, avoiding false-positive or false-negative errors caused by control instability.
Works seamlessly with most commercial ALK primary antibodies: No need for protocol adjustments when switching between antibody brands, reducing the time spent on method optimization.
Adapts to both routine and advanced IHC workflows: Compatible with standard DAB staining for clinical diagnosis and fluorescence-based detection for high-sensitivity research applications, meeting diverse laboratory needs.
Aids in meeting laboratory quality standards: Provides a standardized reference for internal quality control (IQC), helping laboratories comply with medical laboratory management regulations (e.g., ISO 15189) and pass CNAS (China National Accreditation Service for Conformity Assessment) certification.
Facilitates inter-laboratory comparison (ILC): Uniform sample composition ensures consistent control performance across different laboratories, making it easier to identify deviations caused by instrument errors or operational inconsistencies.
Application scenario: Mandatory verification before the first use of a new ALK primary antibody or reagent batch.
Operation steps:
Run the Product in parallel with the new antibody/reagent following the standard ALK IHC protocol.
Evaluate the signal intensity of ALK-positive cells (should meet the antibody’s specified sensitivity) and check for cross-reactivity in ALK-negative cells (no non-specific staining allowed).
Only proceed with clinical sample testing if the validation results meet the quality criteria.
Application scenario: Synchronous quality control for every batch of ALK IHC tests (e.g., daily or per-sample-batch testing).
Operation steps:
Include the Product as a control slide in each IHC run (alongside clinical samples).
After staining, confirm that ALK-positive cells show clear, consistent staining (no signal loss) and ALK-negative cells remain unstained.
If abnormal results occur (e.g., weak positive signal or false negative in controls), stop sample testing immediately and troubleshoot (e.g., check instrument temperature, antibody expiration).
Application scenario: Collaborative quality assessment among multiple laboratories (e.g., hospital laboratory networks or EQA [External Quality Assessment] programs).
Operation steps:
Distribute the same batch of the Product to participating laboratories.
All laboratories test the slide using their in-house ALK IHC protocols.
Compare staining results across laboratories to identify problematic links (e.g., improper antigen retrieval conditions, expired reagents) and standardize operational procedures.
Application scenario: Training for new laboratory technicians on ALK IHC result interpretation.
Operation steps:
Use the Product as a "standard reference slide" to demonstrate typical ALK-positive (e.g., membrane/cytoplasmic staining) and negative (no staining) patterns.
Guide trainees to practice distinguishing true positive signals from non-specific background, helping them master judgment criteria (e.g., staining intensity, localization) quickly.
The Product must be stored at -20°C in a sealed container to prevent moisture absorption. Avoid repeated freeze-thaw cycles—once thawed, use it within 24 hours (if temporarily stored at 2-8°C) to avoid sample degradation. Do not store at room temperature for more than 4 hours.
Yes, the Product requires the same antigen retrieval step as clinical FFPE samples (e.g., heat-induced epitope retrieval with citrate buffer or EDTA buffer). Follow your laboratory’s standard ALK IHC protocol for antigen retrieval—no special adjustments are needed.
It is compatible with both manual and automated IHC staining. For manual staining, ensure consistent timing for antibody incubation and washing (as with clinical samples) to maintain control accuracy. For automated platforms, select the protocol matching your instrument model (e.g., Leica Bond’s ALK-specific program).
First, check if the storage conditions are correct (e.g., whether the slide was exposed to room temperature for too long). If storage is normal, verify the antibody expiration date and antigen retrieval parameters (e.g., temperature, time). If the issue persists, contact technical support for batch-specific quality verification.
No, reuse is not recommended. After one IHC staining cycle, the slide’s cell structure and antigenicity may be damaged, and residual antibodies or chromogens can cause cross-contamination in subsequent tests, leading to inaccurate results.
We possess a large-scale cell resource library, enabling precise selection of ALK-positive cell lines with stable expression and ALK-negative cell lines with no background expression—laying a solid foundation for consistent control performance.
Our mature FFPE slide preparation technology ensures intact cell morphology and preserved antigenicity. Each slide undergoes strict quality checks (e.g., hematoxylin-eosin staining for morphology, IHC validation for signal specificity) before leaving the factory.
Beyond the 2-in-1 ALK FFPE Slide, we offer a full range of IHC reference standards covering CLDN18.2, EGFR, HER2, NTRK, and other key targets. This allows laboratories to source all necessary controls from a single supplier, simplifying procurement and quality management.
We continuously develop reference standards for cutting-edge biomarkers (e.g., emerging immunotherapy targets), helping laboratories keep pace with advances in precision medicine.
Our technical team consists of experienced IHC specialists who provide one-on-one support, including protocol optimization, troubleshooting for abnormal control results, and customized training for laboratory staff.
We offer a product quality guarantee: If the slide fails to meet the specified performance standards under correct storage and use, we provide free replacement or full refund—reducing your risk.
Our IHC reference standards have been adopted by hundreds of pathological laboratories and participated in multiple national EQA programs, demonstrating reliable performance and compliance with clinical diagnostic requirements.
We adhere to strict manufacturing standards, with each product batch accompanied by a detailed quality inspection report—ensuring traceability and transparency for your quality control records.
General information
Name | ALK Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0021 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
IHC staining results

Related Products List
Name | Catalog No. | Details |
ALK Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0022 |
Experimental Procedure
CB-Gene Bio chose ALK as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.
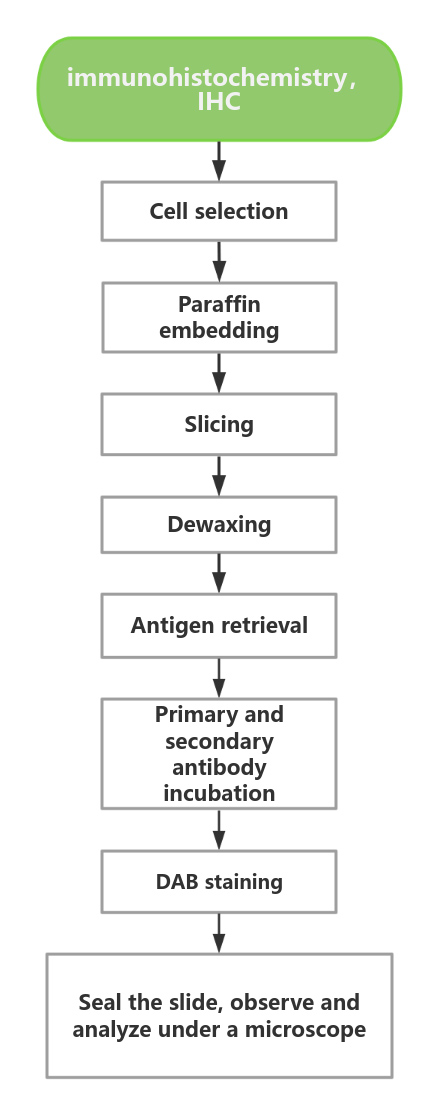
Experimental Procedure
CB-Gene Bio chose ALK as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.

General Information
Name | ALK Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0021 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
General Information
Name | ALK Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0021 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
IHC Staining Results

IHC Staining Results

Related Products List
Name | Catalog No. | Details |
ALK Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0022 |
Related Products List
Name | Catalog No. | Details |
ALK Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0022 |