- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

Views: 0 Author: Site Editor Publish Time: 2025-04-18 Origin: Site
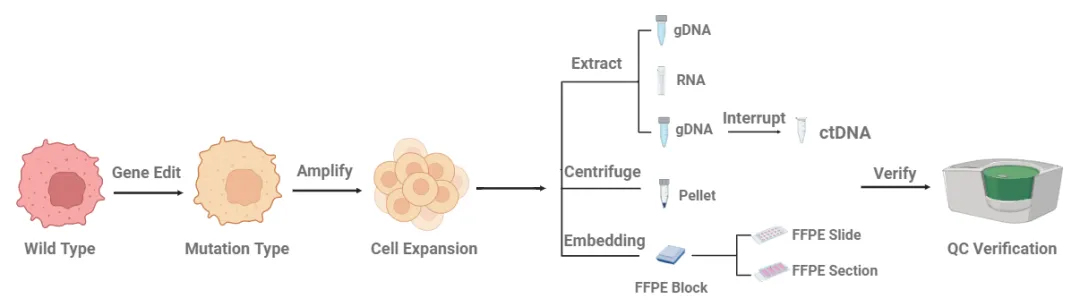
Molecular diagnostic technology and molecular diagnostic standards Molecular diagnostics is a technology that reveals the mechanism of disease occurrence and assists in diagnosis, prognosis assessment and treatment guidance by detecting the molecular characteristics (such as sequence, expression level, methylation status, etc.) of nucleic acids (DNA, RNA) or proteins in biological samples. Its core lies in analyzing disease markers at the molecular level, breaking through the limitations of traditional pathology and biochemical testing, and has high sensitivity (single molecule level), high specificity and early diagnosis potential. The core technologies of molecular diagnosis include PCR (real-time quantitative PCR, digital PCR), gene sequencing (NGS, Sanger sequencing), fluorescence in situ hybridization (FISH), etc., which are used in infectious diseases, personalized tumor treatment (such as EGFR mutation analysis), genetic diseases (such as thalassemia gene diagnosis) and pharmacogenomics. Molecular diagnostic standards are reference materials that have been strictly valued and stability verified. They are used to calibrate instruments, verify method performance and monitor the quality of the entire experimental process to ensure the accuracy, repeatability and cross-platform comparability of test results. Its types include positive/negative controls (to verify detection specificity), quantitative standards (to establish standard curves) and quality control products (to monitor daily stability). Application scenarios of molecular diagnostic standards 1. IVD product development · Performance verification: verify the sensitivity and specificity of the test kit; · Registration application: submit cross-reaction data of standards. 2. Clinical laboratory quality control · Daily quality control: monitor PCR amplification efficiency and NGS sequencing depth uniformity; · Inter-laboratory comparison: transfer the value through standards to achieve mutual recognition of multi-laboratory results. 3. Companion diagnostic development · Companion diagnostic reagent matching: provide specific mutation standards to ensure the accuracy of drug efficacy prediction; · Drug resistance mechanism research: build a drug resistance gene mutation standard library. Preparation process of molecular diagnostic standards Customer application matrix of molecular diagnostic standards in the IVD industry chain Ⅰ. Upstream of IVD industry chain: "ruler" of instrument and raw material manufacturing 1. Customer group: instrument equipment manufacturers (NGS/qPCR/ddPCR platform) Demand scenario: · New equipment performance verification (sensitivity/specificity/repeatability test); · Multi-platform test result consistency calibration. Standard selection: · Cross-platform compatible standards (such as universal DNA quantitative standards); · Extreme sample simulation products (high GC content, fragmented DNA). 2. Customer group: core raw material supplier (enzyme, primer, probe) Demand scenario: · Raw material batch quality control (such as Taq enzyme activity verification); · Competitive product performance benchmarking (testing raw material sensitivity differences through standard products). Standard selection: · Simulated samples containing inhibitors (verification of anti-interference ability); · Low-concentration target standards (detection of raw material extreme sensitivity). Ⅱ. IVD midstream industry chain: "navigator" for kit development 1. Customer group: IVD kit R&D companies Demand scenarios: · R&D stage: target screening and verification (such as new mutation site coverage test); · Performance optimization: sensitivity/specificity verification (LoD/LoQ determination); · Registration application: provide third-party traceable data, such as standard product sets with supporting certificates of analysis (COA); · Production quality inspection: batch release testing, such as internal reference gene standards. Standard product selection: · Multiplex PCR/NGS Panel verification standards; · Complex matrix standards (such as ctDNA simulations in plasma). 2. Reference panel (Panel) developer Demand scenarios: · Cross-reaction verification of multi-target joint detection kits; · Development of clinical sample substitutes (such as FFPE simulation samples). Standard product selection: · Multiplex PCR/NGS Panel verification standards; · Complex matrix standards (such as ctDNA simulations in plasma). Ⅲ. Downstream of IVD Industry Chain: "Guardian" of Clinical Application and Quality Control 1. Customer Group: Pharmaceutical Companies (Development of Companion Diagnostics) Demand Scenarios: · Drug-biomarker matching verification; · Dynamic monitoring of drug-resistant mutations. Core Demands: · Standard products developed simultaneously with CDx kits; · Companion diagnostic clinical trial sample equivalents. 2. Third-party testing agencies (LDT/medical testing institutes) Demand Scenarios: · Daily quality control: intra-batch/inter-batch precision monitoring; · Inter-laboratory quality assessment (EQA): comparison of consistency of results between laboratories; · Detection limit verification: LoD confirmation. 3. Hospital Laboratory/Pathology Department Demand Scenarios: · Verification of new testing projects (such as in-hospital verification of tumor NGS Panel); · Traceability and interpretation of report results. Pain Point Solutions: · Ready-to-use pre-packaged standard products (reduce operational complexity); · Quick interpretation guide (such as Ct value/mutation frequency interpretation threshold). CB-Gene Biotechnology molecular diagnostic standards CB-Gene Biotechnology's tumor molecular diagnostic standards cover a variety of mutation types, including Mutation standards, Fusion standards, CNV (copy number variation) standards, MSI (microsatellite instability) standards, TMB (tumor mutation burden) standards, HRR (homologous recombination repair) standards, etc. Standard products are rich in form, including gDNA (genomic DNA), ctDNA (circulating tumor DNA), FFPE (formalin-fixed paraffin-embedded tissue). Whether it is a single-point standard or a panel standard, CB-Gene Biotechnology strictly controls the quality and improves the customer's highest quality molecular diagnostic standard service.
This category is empty.