- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPN0016
CBPN0016
| Availability: | |
|---|---|
The HER2 Gastric 4-in-1 FFPE Ref Std is a comprehensive reference material designed for validating human epidermal growth factor receptor 2 (HER2) detection workflows in gastric cancer diagnostics. This unique standard combines four distinct HER2 expression and amplification states in a single formalin-fixed, paraffin-embedded (FFPE) block, mimicking the full spectrum of clinical specimens encountered in routine testing. Manufactured using a proprietary fixation protocol that preserves both protein epitopes and nucleic acids, it serves as an essential quality control tool for laboratories performing HER2 testing to guide trastuzumab therapy decisions in gastric adenocarcinoma .
Contains four distinct HER2 phenotypes in spatially separated cores:
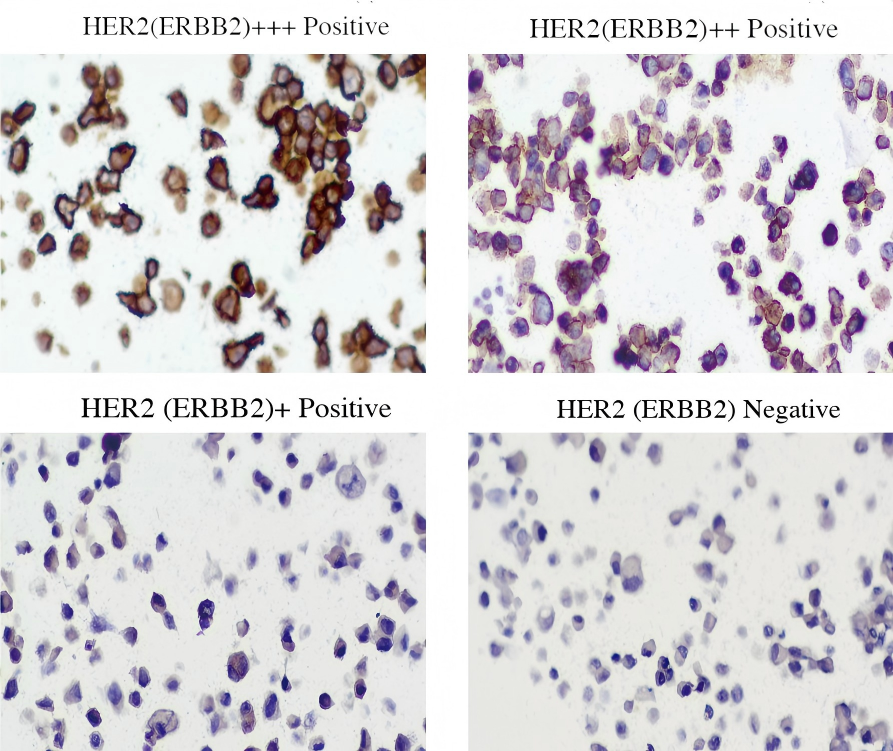
• HER2 0 (negative): <10% immunoreactivity, no amplification
• HER2 1+ (equivocal): weak incomplete membranous staining in ≥10% of cells
• HER2 2+ (equivocal): moderate complete membranous staining in ≥10% of cells
• HER2 3+ (positive): strong complete membranous staining in ≥10% of cells
Each state is verified by both immunohistochemistry (IHC) and in situ hybridization (ISH) .
Processed using 10% neutral buffered formalin fixation (24-hour protocol) and optimized paraffin embedding, the standard maintains histological integrity identical to clinical gastric cancer specimens. This preserves tissue architecture and biomarker expression critical for accurate HER2 assessment .
Each core contains a known number of cells (2 x 107 cells/core)
Section the FFPE block at 5 μm thickness using standard microtomy techniques to ensure sufficient material for both IHC and ISH testing. Deparaffinize using xylene or xylene-free alternatives followed by ethanol rehydration.
Use for validation of HER2 IHC staining protocols by:
• Verifying antibody specificity across expression levels
• Optimizing antigen retrieval conditions (recommended: 95°C in citrate buffer pH 6.0 for 20 minutes)
• Establishing scoring thresholds for each intensity category
• Monitoring staining consistency between reagent lots
Validate HER2 amplification assays by:
• Confirming probe hybridization efficiency
• Verifying signal-to-noise ratios across GCN ranges
• Calibrating HER2/chromosome 17 ratio calculations
• Ensuring proper distinction between amplified and non-amplified states
The combined format allows simultaneous validation of all critical interpretation thresholds in a single run, reducing inter-assay variability and enabling direct comparison between expression states—essential for accurate equivocal case classification .
It facilitates compliance with CAP HER2 testing requirements by providing traceable reference values for both IHC and ISH methods. Comprehensive documentation includes digital images of stained sections and quantitative PCR data .
Yes, the clear distinction between expression states makes it ideal for training laboratory personnel in HER2 scoring criteria, particularly for distinguishing between 1+, 2+, and 3+ categories .
Store unopened blocks at 4°C in a desiccator for up to 36 months. Once sectioned, remaining blocks should be wrapped in paraffin film and stored at 4°C, with sections used within 1 month of cutting .
Name | HER2 gastric 4-in-1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0016 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
IHC staining results
Name | Catalog No. | Details |
HER2 gastric Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0011 |
|
HER2 gastric Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0012 |
|
HER2 gastric 4-in-1 FFPE Block Reference Standard | CBPN0015 |
The HER2 Gastric 4-in-1 FFPE Ref Std is a comprehensive reference material designed for validating human epidermal growth factor receptor 2 (HER2) detection workflows in gastric cancer diagnostics. This unique standard combines four distinct HER2 expression and amplification states in a single formalin-fixed, paraffin-embedded (FFPE) block, mimicking the full spectrum of clinical specimens encountered in routine testing. Manufactured using a proprietary fixation protocol that preserves both protein epitopes and nucleic acids, it serves as an essential quality control tool for laboratories performing HER2 testing to guide trastuzumab therapy decisions in gastric adenocarcinoma .
Contains four distinct HER2 phenotypes in spatially separated cores:
• HER2 0 (negative): <10% immunoreactivity, no amplification
• HER2 1+ (equivocal): weak incomplete membranous staining in ≥10% of cells
• HER2 2+ (equivocal): moderate complete membranous staining in ≥10% of cells
• HER2 3+ (positive): strong complete membranous staining in ≥10% of cells
Each state is verified by both immunohistochemistry (IHC) and in situ hybridization (ISH) .
Processed using 10% neutral buffered formalin fixation (24-hour protocol) and optimized paraffin embedding, the standard maintains histological integrity identical to clinical gastric cancer specimens. This preserves tissue architecture and biomarker expression critical for accurate HER2 assessment .
Each core contains a known number of cells (2 x 107 cells/core)
Section the FFPE block at 5 μm thickness using standard microtomy techniques to ensure sufficient material for both IHC and ISH testing. Deparaffinize using xylene or xylene-free alternatives followed by ethanol rehydration.
Use for validation of HER2 IHC staining protocols by:
• Verifying antibody specificity across expression levels
• Optimizing antigen retrieval conditions (recommended: 95°C in citrate buffer pH 6.0 for 20 minutes)
• Establishing scoring thresholds for each intensity category
• Monitoring staining consistency between reagent lots
Validate HER2 amplification assays by:
• Confirming probe hybridization efficiency
• Verifying signal-to-noise ratios across GCN ranges
• Calibrating HER2/chromosome 17 ratio calculations
• Ensuring proper distinction between amplified and non-amplified states
The combined format allows simultaneous validation of all critical interpretation thresholds in a single run, reducing inter-assay variability and enabling direct comparison between expression states—essential for accurate equivocal case classification .
It facilitates compliance with CAP HER2 testing requirements by providing traceable reference values for both IHC and ISH methods. Comprehensive documentation includes digital images of stained sections and quantitative PCR data .
Yes, the clear distinction between expression states makes it ideal for training laboratory personnel in HER2 scoring criteria, particularly for distinguishing between 1+, 2+, and 3+ categories .
Store unopened blocks at 4°C in a desiccator for up to 36 months. Once sectioned, remaining blocks should be wrapped in paraffin film and stored at 4°C, with sections used within 1 month of cutting .

Name | HER2 gastric 4-in-1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0016 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
IHC staining results

Name | Catalog No. | Details |
HER2 gastric Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0011 |
|
HER2 gastric Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0012 |
|
HER2 gastric 4-in-1 FFPE Block Reference Standard | CBPN0015 |
Experimental Procedure
CB-Gene Bio chose HER2 as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.

Experimental Procedure
CB-Gene Bio chose HER2 as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.

General Information
Name | HER2 gastric 4-in-1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0016 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
General Information
Name | HER2 gastric 4-in-1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0016 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
IHC Staining Results

IHC Staining Results

Related Products List
Name | Catalog No. | Details |
HER2 gastric Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0011 |
|
HER2 gastric Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0012 |
|
HER2 gastric 4-in-1 FFPE Block Reference Standard | CBPN0015 |
Related Products List
Name | Catalog No. | Details |
HER2 gastric Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0011 |
|
HER2 gastric Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0012 |
|
HER2 gastric 4-in-1 FFPE Block Reference Standard | CBPN0015 |