- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPN0001
CBPN0001
| Availability: | |
|---|---|
Background
CLDN 18.2 is a hot target with potential for cancer treatment. Currently, there are four main types of targeted drugs approved for clinical trials: monoclonal antibodies, ADCs, CAR-T and bispecific antibodies (BsAb). Claudin18.2-targeted monoclonal antibodies, ADCs, BsAbs and CAR-T therapies are mainly based on IHC screening of patients with positive/high expression of Claudin18.2 in tumors. The results of multiple current clinical trials suggest that targeted Claudin18.2 therapy is more effective in cancer patient subgroups with higher Claudin18.2 positivity rates detected by IHC.
There is currently no gold standard for the detection of Claudin18.2, and its interpretation criteria vary in different studies. Since Zolbetuximab is the only drug to enter Phase III clinical trials, many drugs refer to its positive results as the inclusion criteria.
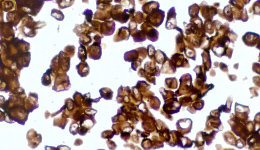
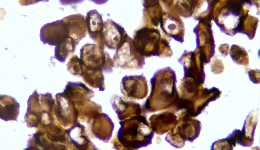
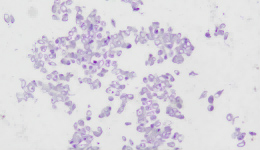
① Determine the intensity of CLDN18.2 protein staining on the tumor cell membrane, and the percentage of cancer cells with complete, basolateral or lateral membrane staining.
② CLDN18.2 positive: any tumor with a staining intensity of 2+ or 3+ (total) in ≥40% of tumor cells.
Therefore, accurate screening of Claudin18.2 expression by IHC is crucial for the reliability of clinical trial data and the effectiveness of precision medicine.
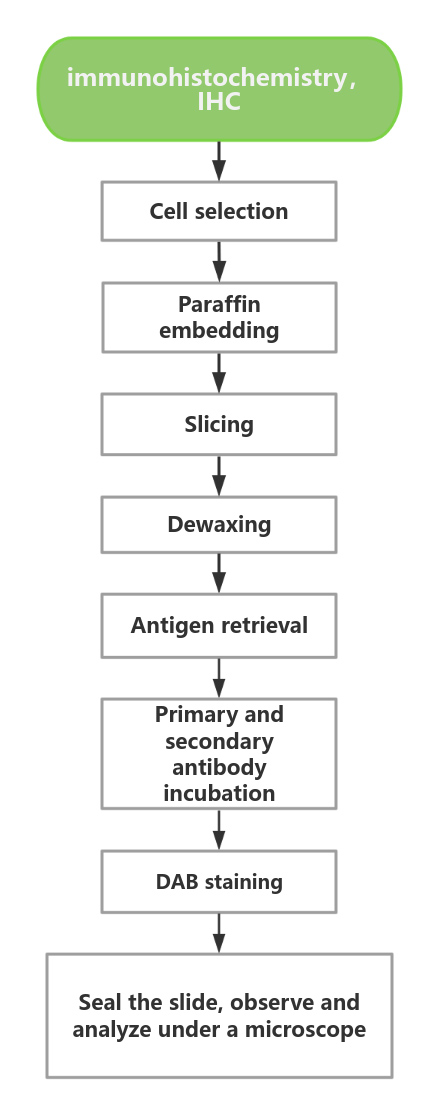
Experimental Procedure
Since the middle of the 20th century, immunohistochemistry (IHC) technology has entered the stage of clinical pathological diagnosis. Today, IHC testing has become an indispensable technical means, playing an important role in pathological diagnosis, especially in the diagnosis of tumors. However, the IHC method is like a double-edged sword. If the quality control is unqualified, it will cause false positives or false negatives, leading to misdiagnosis and missed diagnosis of pathology, giving wrong clinical information, and ultimately delaying the treatment of patients and causing serious consequences. Therefore, setting up controls during immunohistochemical staining is one of the most important measures for staining quality control, especially negative and positive controls.
In 2014 and 2015, the International Special Expert Committee made expert recommendations on the classification and standard definition of diagnostic immunohistochemical controls, the interpretation of negative control results and the selection and application.
Based on the important role of IHC in clinical pathological diagnosis, it is particularly important to establish a scientific, standardized and refined immunohistochemical quality control system. Good immunohistochemical staining sections are the basis and prerequisite for correctly judging staining results. Since there are many steps or links in the immunohistochemical staining process, each step or link may affect the final result of the staining.
CB-Gene Bio chose CLDN18.2 as the research object and launched two standard products: wax block and fishing slice.
General information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 4μm |
Example |
|
IHC staining results
 |  |  |
 | 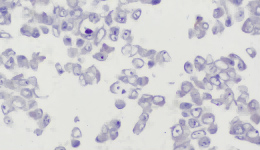 | 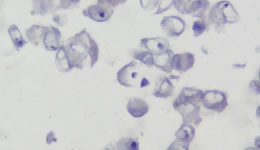 |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |
Background
CLDN 18.2 is a hot target with potential for cancer treatment. Currently, there are four main types of targeted drugs approved for clinical trials: monoclonal antibodies, ADCs, CAR-T and bispecific antibodies (BsAb). Claudin18.2-targeted monoclonal antibodies, ADCs, BsAbs and CAR-T therapies are mainly based on IHC screening of patients with positive/high expression of Claudin18.2 in tumors. The results of multiple current clinical trials suggest that targeted Claudin18.2 therapy is more effective in cancer patient subgroups with higher Claudin18.2 positivity rates detected by IHC.
There is currently no gold standard for the detection of Claudin18.2, and its interpretation criteria vary in different studies. Since Zolbetuximab is the only drug to enter Phase III clinical trials, many drugs refer to its positive results as the inclusion criteria.
① Determine the intensity of CLDN18.2 protein staining on the tumor cell membrane, and the percentage of cancer cells with complete, basolateral or lateral membrane staining.
② CLDN18.2 positive: any tumor with a staining intensity of 2+ or 3+ (total) in ≥40% of tumor cells.
Therefore, accurate screening of Claudin18.2 expression by IHC is crucial for the reliability of clinical trial data and the effectiveness of precision medicine.
Experimental Procedure
Since the middle of the 20th century, immunohistochemistry (IHC) technology has entered the stage of clinical pathological diagnosis. Today, IHC testing has become an indispensable technical means, playing an important role in pathological diagnosis, especially in the diagnosis of tumors. However, the IHC method is like a double-edged sword. If the quality control is unqualified, it will cause false positives or false negatives, leading to misdiagnosis and missed diagnosis of pathology, giving wrong clinical information, and ultimately delaying the treatment of patients and causing serious consequences. Therefore, setting up controls during immunohistochemical staining is one of the most important measures for staining quality control, especially negative and positive controls.
In 2014 and 2015, the International Special Expert Committee made expert recommendations on the classification and standard definition of diagnostic immunohistochemical controls, the interpretation of negative control results and the selection and application.
Based on the important role of IHC in clinical pathological diagnosis, it is particularly important to establish a scientific, standardized and refined immunohistochemical quality control system. Good immunohistochemical staining sections are the basis and prerequisite for correctly judging staining results. Since there are many steps or links in the immunohistochemical staining process, each step or link may affect the final result of the staining.
CB-Gene Bio chose CLDN18.2 as the research object and launched two standard products: wax block and fishing slice.

General information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 4μm |
Example |
|
IHC staining results
 |  |  |
 |  |  |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |
General Information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
General Information
Name | CLDN18.2 +++ FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0001 |
Format | FFPE Microscope Slide |
Size | 4μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 4μm |
Example |
|
Technical Data
Slice thickness | 4μm |
Example |
|
IHC Staining Results
 |  |  |
 |  |  |
IHC Staining Results
 |  |  |
 |  |  |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |
Related Products List
Name | Catalog No. | Details |
CLDN18.2 +++ FFPE Microscope Slide Reference Standard | CBPN0001 |
|
CLDN18.2 +++ FFPE Block Reference Standard | CBPN0002 | |
CLDN18.2 ++ FFPE Microscope Slide Reference Standard | CBPN0003 |
|
CLDN18.2 ++ FFPE Block Reference Standard | CBPN0004 | |
CLDN18.2 + FFPE Microscope Slide Reference Standard | CBPN0005 |
|
CLDN18.2 + FFPE Block Reference Standard | CBPN0006 | |
CLDN18.2 Negative FFPE Microscope Slide Reference Standard | CBPN0007 |
|
CLDN18.2 Negative FFPE Block Reference Standard | CBPN0008 |
|
CLDN18.2 Positive Negative 2-in-1 FFPE Block Reference Standard | CBPN0009 |
|
CLDN18.2 Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard | CBPN0010 |
|
CLDN18.2 4-in-1 FFPE Block Reference Standard | CBPN0013 | |
CLDN18.2 4-in-1 FFPE Microscope Slide Reference Standard | CBPN0014 |