- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPN0019
CBPN0019
| Availability: | |
|---|---|
The 2-in-1 EGFR FFPE Slide Ref Std is a specialized reference material designed for validating epidermal growth factor receptor (EGFR) mutation detection workflows in non-small cell lung cancer (NSCLC) diagnostics. This pre-cut FFPE slide contains both negative and positive samples, and different expression levels can be observed using IHC staining.This method provides a ready-to-use tool for guiding the accuracy of molecular assays in selecting tyrosine kinase inhibitor (TKI) treatments.Manufactured to preserve both histological morphology and nucleic acid integrity, this standard addresses the critical need for consistent controls in EGFR testing, where accurate mutation detection directly impacts patient treatment decisions .
By detecting the expression of specific proteins in tissue samples using EGFR, pathologists can accurately classify and diagnose diseases, and determine the primary site of tumors.
Each slide contains 5 μm sections, which maintain a uniform cell density (2.5 x 107 cells/section) and preserve tissue structure for simultaneous histopathological evaluation.
Prior to molecular testing, stain one section with Hematoxylin and Eosin (H&E) to:
• Confirm tissue morphology preservation
• Verify section integrity
• Establish appropriate regions for microdissection if needed
Yes, the optimized fixation protocol preserves both EGFR protein epitopes and genomic DNA integrity, allowing simultaneous validation of IHC for protein expression and molecular methods for mutation detection .
Each slide contains sufficient material for 3-4 independent extractions, enabling duplicate or triplicate testing across different assay runs or platforms .
Store slides at 4°C in a slide box with desiccant for up to 24 months unopened. Once opened, use remaining sections within 4 weeks and maintain storage conditions to prevent tissue degradation .
General information
Name | EGFR Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0019 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
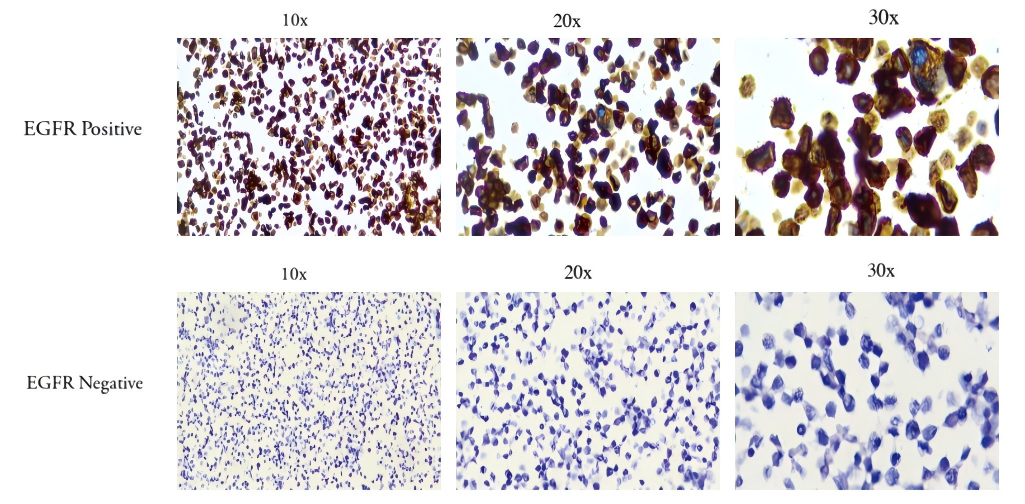
IHC staining results
Related Products List
Name | Catalog No. | Details |
EGFR Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0020 |
The 2-in-1 EGFR FFPE Slide Ref Std is a specialized reference material designed for validating epidermal growth factor receptor (EGFR) mutation detection workflows in non-small cell lung cancer (NSCLC) diagnostics. This pre-cut FFPE slide contains both negative and positive samples, and different expression levels can be observed using IHC staining.This method provides a ready-to-use tool for guiding the accuracy of molecular assays in selecting tyrosine kinase inhibitor (TKI) treatments.Manufactured to preserve both histological morphology and nucleic acid integrity, this standard addresses the critical need for consistent controls in EGFR testing, where accurate mutation detection directly impacts patient treatment decisions .
By detecting the expression of specific proteins in tissue samples using EGFR, pathologists can accurately classify and diagnose diseases, and determine the primary site of tumors.
Each slide contains 5 μm sections, which maintain a uniform cell density (2.5 x 107 cells/section) and preserve tissue structure for simultaneous histopathological evaluation.
Prior to molecular testing, stain one section with Hematoxylin and Eosin (H&E) to:
• Confirm tissue morphology preservation
• Verify section integrity
• Establish appropriate regions for microdissection if needed
Yes, the optimized fixation protocol preserves both EGFR protein epitopes and genomic DNA integrity, allowing simultaneous validation of IHC for protein expression and molecular methods for mutation detection .
Each slide contains sufficient material for 3-4 independent extractions, enabling duplicate or triplicate testing across different assay runs or platforms .
Store slides at 4°C in a slide box with desiccant for up to 24 months unopened. Once opened, use remaining sections within 4 weeks and maintain storage conditions to prevent tissue degradation .

General information
Name | EGFR Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0019 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
Technical Data
Slice thickness | 5μm |
IHC staining results

Related Products List
Name | Catalog No. | Details |
EGFR Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0020 |
Experimental Procedure
CB-Gene Bio chose EGFR as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.

Experimental Procedure
CB-Gene Bio chose EGFR as the research object and launched two standard products: FFPE Block and FFPE Microscope Slide.

General Information
Name | EGFR Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0019 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
General Information
Name | EGFR Positive Negative 2 in 1 FFPE Microscope Slide Reference Standard |
Cat. No. | CBPN0019 |
Format | FFPE Microscope Slide |
Size | 5μm/section |
Intended Use | Research Use Only |
Buffer | Tris-EDTA |
Storage Conditions | -25~-15℃ |
Expiry | 36 months from the date of manufacture |
IHC Staining Results

IHC Staining Results

Related Products List
Name | Catalog No. | Details |
EGFR Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0020 |
Related Products List
Name | Catalog No. | Details |
EGFR Positive Negative 2 in 1 FFPE Block Reference Standard | CBPN0020 |