- Home
- Products
- Services
- Resources
- About Us
- News
- Contact

loading
CBPJ0005
CBPJ0005
| Availability: | |
|---|---|
The 1μg Genomic DNA 47,XXY Ref Std for NIPT is a highly characterized reference material designed specifically for validating non-invasive prenatal testing (NIPT) workflows for detecting sex chromosome aneuploidies. This standard contains genomic DNA from a confirmed 47,XXY karyotype (Klinefelter syndrome), providing a precise reference for calibrating NIPT assays that screen for fetal sex chromosome abnormalities. With a total mass of 1μg at approximately 40 ng/μL concentration, it mimics the fetal fraction characteristics of cell-free DNA in maternal plasma, enabling accurate validation of analytical performance in NIPT platforms. Manufactured to reflect the genomic complexity of clinical specimens, this standard is an essential quality control tool for laboratories performing prenatal aneuploidy screening.
Processed to mimic cell-free DNA characteristics:
• Fragment size distribution of 160-180 bp (matching cfDNA in plasma)
• Compatible with NGS library preparation protocols for low-input DNA
Precisely calibrated for NIPT validation:
• 1μg total DNA per vial (25 μL at approximately 40 ng/μL)
• X chromosome copy number: 2.0 ± 0.1
• Y chromosome copy number: 1.0 ± 0.1
• Autosome copy number: 2.0 ± 0.1 (verified for chromosomes 13, 18, 21)
• Genome-wide GC content matching human reference (41-42%)
Use to validate NIPT performance parameters:
• Sex chromosome aneuploidy detection accuracy
• Fetal fraction estimation algorithms
• Chromosome-specific bias correction
Incorporate into method validation to:
• Verify 47,XXY detection across sequencing depths
• Establish analytical sensitivity (>99%) and specificity (>99.5%)
• Monitor inter-run variability in chromosome dosage calling
• Validate bioinformatics pipelines for ploidy estimation
Store unopened vials at -80°C for up to 24 months from manufacture. After first use, aliquot remaining DNA into single-use volumes (1-5 μL) and store at -80°C. Avoid freeze-thaw cycles (maximum 2 cycles). Thaw on ice for 10 minutes before use and mix gently by pipetting. Do not vortex.
Klinefelter syndrome (47,XXY) is the most common sex chromosome aneuploidy, affecting 1 in 500-1,000 male births. Early detection enables anticipatory guidance regarding fertility, hormonal development, and educational needs. NIPT screening has shown >95% detection rate for 47,XXY when using validated assays .
It provides a well-characterized reference with known karyotype, enabling laboratories to verify their ability to correctly identify sex chromosome aneuploidies and distinguish them from normal male (46,XY) and female (46,XX) profiles. This is critical for reducing false positive and false negative rates .
Yes, the standard is validated for use with all major NIPT platforms including Illumina NovaSeq, Thermo Fisher Ion Proton, and BGI MGISEQ systems.
By diluting with female genomic DNA (46,XX), laboratories can create mixtures simulating fetal fractions from 1-20%, enabling validation of fetal fraction calculation algorithms and establishing minimum required fetal fraction for reliable 47,XXY detection.
Product Information | Klinefelter Syndrome (47,XXY) Reference Standard |
Catalog ID | CBPJ0005 |
Format | Genomic DNA |
Intended Use | Research Use Only |
Unit Size | 1ug |
Sanger sequencing | Download |
Storage | 2-8°C |
Expiry | 36 months from the date of manufacture |
Technical Data
Mutation | Klinefelter Syndrome |
Karyotype | 47,XXY |
Representative Data
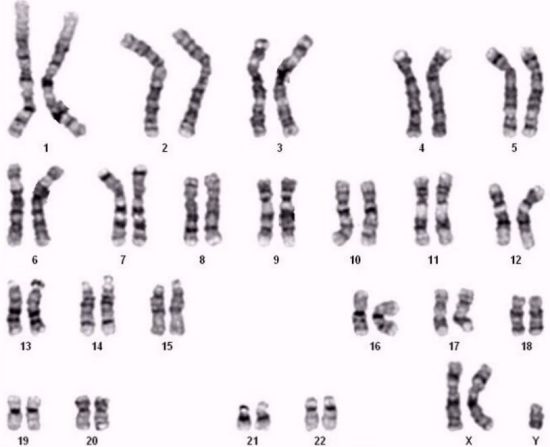
The 1μg Genomic DNA 47,XXY Ref Std for NIPT is a highly characterized reference material designed specifically for validating non-invasive prenatal testing (NIPT) workflows for detecting sex chromosome aneuploidies. This standard contains genomic DNA from a confirmed 47,XXY karyotype (Klinefelter syndrome), providing a precise reference for calibrating NIPT assays that screen for fetal sex chromosome abnormalities. With a total mass of 1μg at approximately 40 ng/μL concentration, it mimics the fetal fraction characteristics of cell-free DNA in maternal plasma, enabling accurate validation of analytical performance in NIPT platforms. Manufactured to reflect the genomic complexity of clinical specimens, this standard is an essential quality control tool for laboratories performing prenatal aneuploidy screening.
Processed to mimic cell-free DNA characteristics:
• Fragment size distribution of 160-180 bp (matching cfDNA in plasma)
• Compatible with NGS library preparation protocols for low-input DNA
Precisely calibrated for NIPT validation:
• 1μg total DNA per vial (25 μL at approximately 40 ng/μL)
• X chromosome copy number: 2.0 ± 0.1
• Y chromosome copy number: 1.0 ± 0.1
• Autosome copy number: 2.0 ± 0.1 (verified for chromosomes 13, 18, 21)
• Genome-wide GC content matching human reference (41-42%)
Use to validate NIPT performance parameters:
• Sex chromosome aneuploidy detection accuracy
• Fetal fraction estimation algorithms
• Chromosome-specific bias correction
Incorporate into method validation to:
• Verify 47,XXY detection across sequencing depths
• Establish analytical sensitivity (>99%) and specificity (>99.5%)
• Monitor inter-run variability in chromosome dosage calling
• Validate bioinformatics pipelines for ploidy estimation
Store unopened vials at -80°C for up to 24 months from manufacture. After first use, aliquot remaining DNA into single-use volumes (1-5 μL) and store at -80°C. Avoid freeze-thaw cycles (maximum 2 cycles). Thaw on ice for 10 minutes before use and mix gently by pipetting. Do not vortex.
Klinefelter syndrome (47,XXY) is the most common sex chromosome aneuploidy, affecting 1 in 500-1,000 male births. Early detection enables anticipatory guidance regarding fertility, hormonal development, and educational needs. NIPT screening has shown >95% detection rate for 47,XXY when using validated assays .
It provides a well-characterized reference with known karyotype, enabling laboratories to verify their ability to correctly identify sex chromosome aneuploidies and distinguish them from normal male (46,XY) and female (46,XX) profiles. This is critical for reducing false positive and false negative rates .
Yes, the standard is validated for use with all major NIPT platforms including Illumina NovaSeq, Thermo Fisher Ion Proton, and BGI MGISEQ systems.
By diluting with female genomic DNA (46,XX), laboratories can create mixtures simulating fetal fractions from 1-20%, enabling validation of fetal fraction calculation algorithms and establishing minimum required fetal fraction for reliable 47,XXY detection.
Product Information | Klinefelter Syndrome (47,XXY) Reference Standard |
Catalog ID | CBPJ0005 |
Format | Genomic DNA |
Intended Use | Research Use Only |
Unit Size | 1ug |
Sanger sequencing | Download |
Storage | 2-8°C |
Expiry | 36 months from the date of manufacture |
Technical Data
Mutation | Klinefelter Syndrome |
Karyotype | 47,XXY |
Representative Data

General Information
Product Information | Klinefelter Syndrome (47,XXY) Reference Standard |
Catalog ID | CBPJ0005 |
Format | Genomic DNA |
Intended Use | Research Use Only |
Unit Size | 1ug |
Sanger sequencing | Download |
Storage | 2-8°C |
Expiry | 36 months from the date of manufacture |
General Information
Product Information | Klinefelter Syndrome (47,XXY) Reference Standard |
Catalog ID | CBPJ0005 |
Format | Genomic DNA |
Intended Use | Research Use Only |
Unit Size | 1ug |
Sanger sequencing | Download |
Storage | 2-8°C |
Expiry | 36 months from the date of manufacture |
Technical Data
Mutation | Klinefelter Syndrome |
Karyotype | 47,XXY |
Technical Data
Mutation | Klinefelter Syndrome |
Karyotype | 47,XXY |
Product Application

Product Application
